Note: This issue is evolving. We will update this post as news comes out. This post was updated on December 16, 2020.
What is the current guidance for Pfizer/BioNTech vaccine administration in the US for people with allergies?
The U.S. Centers for Disease Control and Prevention (CDC) has issued guidance for people with allergies and whether they should receive the Pfizer/BioNTech vaccine for COVID-19. This guidance was issued when the U.S. Food and Drug Administration (FDA) approved the vaccine Dec. 11 for emergency use in Americans ages 16 and over.
The guidance states the following:
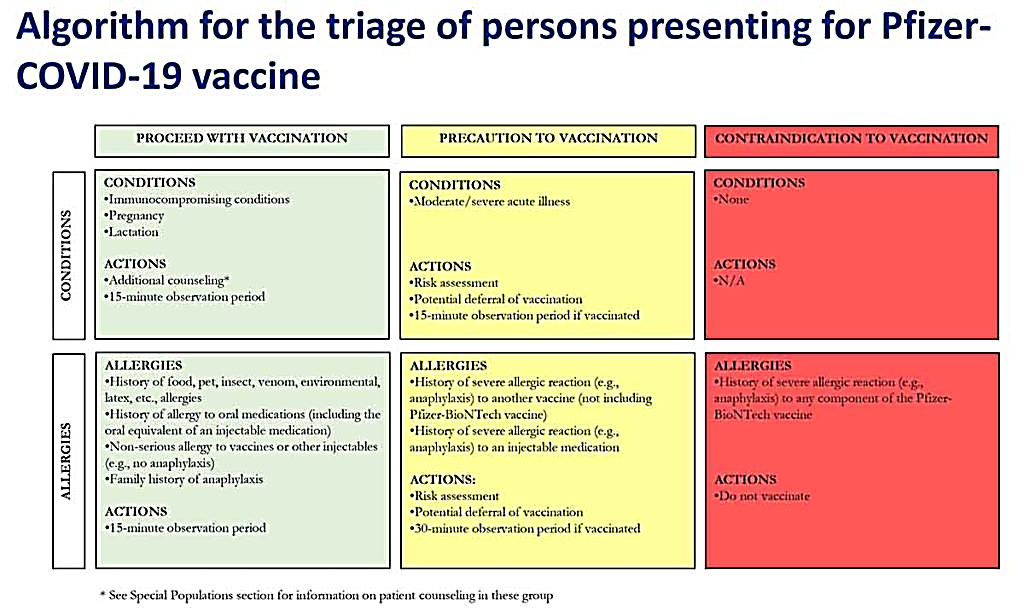
People who should receive the Pfizer/BioNTech vaccine
- Anyone with a history of food, insect venom, oral medications, environmental or latex allergies
- Anyone who has a non-serious allergy to vaccines or other injected medications.
- Anyone with a family history of anaphylaxis
Must undergo a 15-minute observation period after receiving the vaccine to ensure there’s no severe allergic reaction.
People who should take caution with the vaccine along with a 30-minute observation period after vaccination
- Anyone with a history of anaphylaxis to another vaccine or an injected medication
Discuss risks of vaccine and whether to wait to receive the vaccine; must undergo a 30-minute observation period after receiving the vaccine to ensure there’s no severe allergic reaction.
People who should not receive the vaccine:
- Anyone with a history of anaphylaxis to any component of the Pfizer/BioNTech vaccine.
What is the algorithm use to decide if people should receive the Pfizer/BioNTech COVID-19 vaccine?
What is known regarding the COVID-19 vaccine allergy warning from the United Kingdom?
The CDC guidance came after health officials in the United Kingdom issued a warning for the Pfizer/BioNTech vaccine. UK health officials warned that people with a significant history of allergic reactions to food, vaccines or medicines should not be given the vaccine. The warning came after two healthcare workers experienced anaphylaxis after receiving the vaccine. Both had a history of allergic reactions and used epinephrine to treat symptoms.
More research is needed to investigate the two reported cases of anaphylaxis, as well as the thousands of people who received the Pfizer/BioNTech vaccine in the UK and United States during clinical trials.
People with a history of severe allergic reactions to vaccines were not part of Pfizer/BioNTech clinical trials earlier this year, notes allergist Purvi Parikh, MD, national spokesperson for Allergy & Asthma Network. “People with other types of allergies participated in the clinical trials and likely tolerated the vaccine with no issue,” she says.
UK health officials state that people with food allergies or a single medication allergy and no history of anaphylaxis are not at significantly higher risk of experiencing an allergic reaction to the vaccine. In addition, if a person experiences anaphylaxis as a result of the vaccine, they should not receive a second dose, UK health officials say.
How common is anaphylaxis to a vaccine?
Anaphylactic reactions to vaccines are extremely rare. They can occur due to the presence of a particular ingredient or preservative, such as egg or gelatin, however, the amount is so tiny that it is unlikely to trigger a severe allergic reaction.
Does the Pfizer/BioNTech COVID-19 vaccine contain egg?
There is no link to the Pfizer/BioNTech vaccine and concerns of egg allergy and flu vaccine, as has been falsely reported by some media outlets.
Does the Pfizer/BioNTech COVID-19 vaccine contain any preservatives?
The Pfizer-BioNTech COVID-19 vaccine does not contain any preservatives that may trigger an allergic reaction.
Do the Pfizer/BioNTech COVID-19 vaccine stoppers contain any natural rubber latex?
The vial stoppers are not made with natural rubber latex, so the Pfizer/BioNTech COVID-19 vaccine is safe for people with latex allergy.
Does the Pfizer/BioNTech COVID-19 vaccine contain PEG?
Some reports suggest that polyethylene glycol (PEG), a compound that helps the vaccine access cells, may have played a role in the two cases of anaphylaxis. Allergic reactions to PEG are also considered rare. The American College of Allergy, Asthma & Immunology (ACAAI) recommends that people with a known history of severe allergic reactions to PEG should not receive the vaccine.
The two reported cases of anaphylaxis in the UK should not deter anyone from seeking out the COVID-19 vaccine.
COVID-19 vaccines from other pharmaceutical companies are coming soon. Similar allergy concerns may arise with these new vaccines. We urge people with a history of anaphylaxis to consult with a board-certified allergist if they are concerned about the COVID-19 vaccine. Always keep with you two epinephrine auto-injectors if you are at risk for anaphylaxis.
